In physics, when we talk about a particle carrying a charge equal to 100 times the charge of an electron, we’re referring to a situation where the particle holds an electric charge that is 100× greater than the elementary charge (e), which is the charge of a single proton or the magnitude of an electron’s charge.
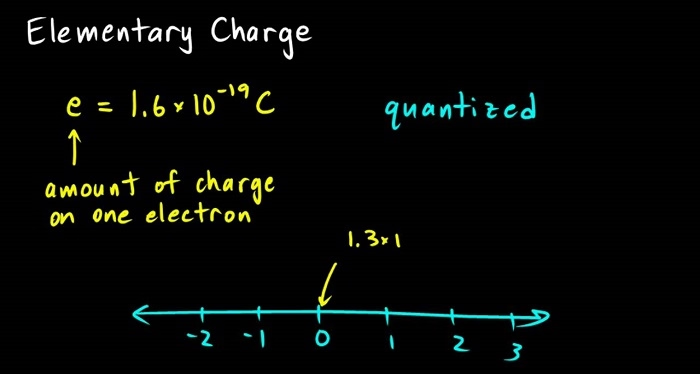
What Is the Elementary Charge?
The elementary charge (e) is:
e=1.6×10−19 C (coulombs)e = 1.6 \times 10^{-19} \text{ C (coulombs)}e=1.6×10−19 C (coulombs)
It represents the smallest unit of electric charge in nature, carried by a proton (+) or electron (–).
Calculating the Charge of the Particle
If a particle carries 100 times this charge:
Q=100×e=100×(1.6×10−19)=1.6×10−17 CQ = 100 \times e = 100 \times (1.6 \times 10^{-19}) = 1.6 \times 10^{-17} \text{ C}Q=100×e=100×(1.6×10−19)=1.6×10−17 C
This value could be positive or negative, depending on whether the particle is positively charged (like a heavy ion) or negatively charged (like an electron cloud or charged droplet).
What Does It Mean in Practice?
- High Charge Density: A particle with this much charge would interact very strongly with electric and magnetic fields.
- Coulomb Force: The electrostatic force it experiences or exerts would be 100× stronger than that of a single electron.
- Seen in Accelerators or Plasmas: Such particles might appear in ion beams, plasma physics, or nuclear reactions where heavy ions carry multiple charges.
Applications in Science
- Particle Physics: Multiply charged particles are common in ion accelerators.
- Electrostatics: Helps in calculating force, potential energy, and field intensity.
- Plasma & Astrophysics: Charged particles in stars and reactors can carry large multiples of e.
💡 Tip: To find the force or energy involving such a particle, just use its total charge Q=1.6×10−17 CQ = 1.6 \times 10^{-17} \text{ C}Q=1.6×10−17 C in standard electrostatic formulas.