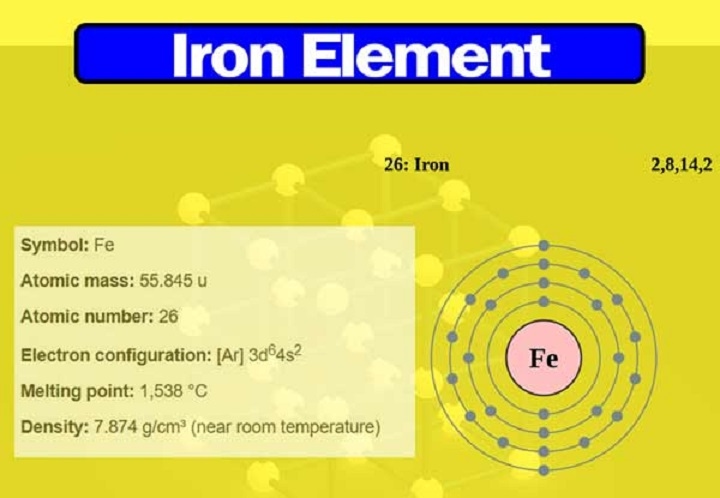
The phrase iron valency and symbol defines the chemical notation of iron and how the substance is able to form other partnerships with other elements using different oxidation numbers. Iron has one of the richest deposits and is largely used in the world. It has the chemical symbol Fe (derived, in turn, from the Latin word Ferrum) and has multiple valencies [-2 and -3] as well but generally +2 and +3. Appreciation of the symbol and valency of iron by students of chemistry, researchers, and others in metallurgy, engineering, and the industry, is very essential.
This paper gives details of the definition, history, chemical properties, compounds, and uses of iron. By means of examples and tables, we give a full picture of the issue of the necessity of iron both in academic and in practical study.
What Does The Iron Symbol And The Valency Mean?
The iron sign is that of Fe, which denotes the element in chemical equations, formulas and other literature found in science. The valency of iron is variable, the most frequent valency is +2 (ferrous) and +3 (ferric). By taking on different numbers of valencies, iron can be used to create a broad range of different compounds that have diverse and varied chemical properties and experimental uses.
The valency of iron plays an important part in the interpretation of reactions, prediction of products and the explanation of how iron can combine with other elements to form such compounds as iron(II) oxide (FeO), iron(III) chloride (FeCl 3 ) and ferrites found in electronic applications.
The Electron Configuration of Iron
Iron is a transition metal whose physico-chemical properties are very special. Its atomic structure and valency makes it very reactive and multi-purposed in chemical reactions.
| Property | Value | Significance |
| Symbol | Fe | Standard chemical representation |
| Atomic Number | 26 | Number of protons in nucleus |
| Atomic Mass | 55.85 u | Mass of a single atom |
| Valency | +2, +3 | Forms compounds in multiple oxidation states |
| Group | 8 | Transition metals group |
| Period | 4 | Row in the periodic table |
| Density | 7.87 g/cm³ | Heavy and malleable metal |
| Melting Point | 1538 °C | High thermal resistance |
| Boiling Point | 2862 °C | Suitable for high-temperature applications |
The variability of valency renders iron useful in a variety of oxides, halides, sulfides, as well as the complex ions of medicines going hand in hand with chemistry and industry.
These Common Compounds of Iron and their Symbols
Because of its variable valency, iron forms many compounds. The symbol of iron (Fe) and the valency is written to write the chemical formulas precisely.
| Compound | Chemical Formula | Iron Valency | Use |
| Iron(II) Oxide | FeO | +2 | Pigments, steel manufacturing |
| Iron(III) Oxide | Fe₂O₃ | +3 | Rust, pigments, magnetic materials |
| Iron(II) Sulfate | FeSO₄ | +2 | Fertilizers, water treatment |
| Iron(III) Chloride | FeCl₃ | +3 | Water purification, etching |
| Ferrite | MFe₂O₄ | +2/+3 | Electronics, magnetic storage |
These are examples of how complex the chemical form of iron can be so that it can be vital in laboratory and industrial settings.
Chemistry Structure of Iron Compounds
Iron has a wide range of structures based upon oxidation state, bond types and coordination.
| Compound | Structure | Bonding Type | Special Feature |
| FeO | Cubic lattice | Ionic | Forms black crystalline solid |
| Fe₂O₃ | Hexagonal lattice | Ionic | Commonly known as rust |
| FeSO₄·7H₂O | Monoclinic lattice | Ionic + hydrogen bonding | Blue crystalline hydrate |
| FeCl₃ | Trigonal planar | Covalent | Strong Lewis acid, soluble in water |
| Ferrite | Spinel structure | Ionic-covalent | Magnetic and stable |
The structure and bonding assist in explaining why iron has the chemical behavior, reactivity and industrial usefulness it does.
Application and uses of iron compounds
Iron and its products are highly used in the industries, agriculture, medicine, and technology sectors.
| Compound / Form | Application | Industry / Use |
| Metallic Iron | Construction, machinery | Building infrastructure and tools |
| Iron(II) Sulfate | Fertilizer | Agriculture and horticulture |
| Iron(III) Oxide | Pigments, magnetic materials | Paint, ceramics, electronics |
| Iron(III) Chloride | Water treatment | Purification of drinking water |
| Ferrites | Electronics | Magnetic cores, inductors, transformers |
The variability in iron, in various forms, indicates how significant iron is in our day-to-day lifestyle at the same time in advanced technology.
The properties of Iron are physical and chemical
Iron has unique attributes that can be used in construction and manufacturing industries as well as in chemicals.
| Property | Value | Significance |
| Appearance | Lustrous metallic gray | Attractive and durable for construction |
| Conductivity | Good conductor of heat and electricity | Useful in electrical applications |
| Malleability | High | Can be shaped into sheets and wires |
| Reactivity | Forms oxides and salts | Participates in redox reactions and alloying |
| Magnetic Property | Ferromagnetic | Used in motors, transformers, and storage devices |
| Density | 7.87 g/cm³ | Heavy and stable for structural use |
Such characteristics supplement the multiple chemical uses of iron, thus it has formed a basis of modern industry.
Iron Reactions and Valency Role
The valency of iron determines its chemical reactions. The ferrous + 2 and ferric + 3 have diverse reactivities towards elements and compounds.
| Reaction | Reactants | Products | Iron Valency |
| Oxidation to Ferric Oxide | 4Fe + 3O₂ → 2Fe₂O₃ | Rust formation | +3 |
| Formation of Ferrous Sulfate | Fe + H₂SO₄ → FeSO₄ + H₂ | +2 | |
| Reaction with Hydrochloric Acid | Fe + 2HCl → FeCl₂ + H₂ | +2 | |
| Formation of Ferric Chloride | 2Fe + 3Cl₂ → 2FeCl₃ | +3 | |
| Complex Formation | Fe³⁺ + 6CN⁻ → [Fe(CN)₆]³⁻ | +3 |
These responses show why compounds depend on the careful comprehension of iron valency in the context of chemical synthesis and production.
FAQs Iron Valency and Symbol
What is the symbol iron?
The chemical symbol associated with iron is Fe, which has been derived with the Latin name- Ferrum.
How many valency electrons are there in Fe?
Iron can have a number of valencies, most commonly +2 (ferrous) and +3 (ferric).
Which are common compounds of iron?
Examples of common compounds are iron(II) oxide (FeO), iron(III) oxide (Fe 2 O 3 ), iron ( II ) sulfate (FeSO 4 ) and iron(III) chloride (FeCl 3 ) and ferrites.
Why did iron occur inAB wholesome and unadulterated, and why did its valency manifest itself in multiple ways?
Industrially, iron can give up 2 or 3 electrons in its 4s and 3d orbitals (thus assuming an oxidation state of +2 or +3).
In what life do we use iron?
Iron finds application in the construction sector, in construction, machinery, electronics, pigments, water treatment and magnetic devices.
Knowledge of iron valency and symbol is essential to chemistry students, research and industrial personnel in the metal manufacturing and industry. Fe is used universally as a shorthand term describing iron and its valency (+2 and +3) informs us of the ranges of chemical activity and combinations available. The compounds of iron, e.g. FeO, Fe 2O 3, FeSO 4, ferrites prove the applicability of iron in industries, agriculture and technology. Familiarity with the symbol, valency, properties and reactions of iron are fundamental to making predictions of how it will behave, practical use in designing industrial processes, and making the best use of its properties.