The magnesium ion is one of the most essential ions in both chemistry and biology. Whether you’re studying chemical bonding, human physiology, or water quality, understanding how magnesium is represented as an ion is a foundational concept. The symbol of magnesium ion is more than just a notation—it’s a key to understanding how this element behaves, interacts, and contributes to various natural and engineered systems.
What Is a Magnesium Ion?
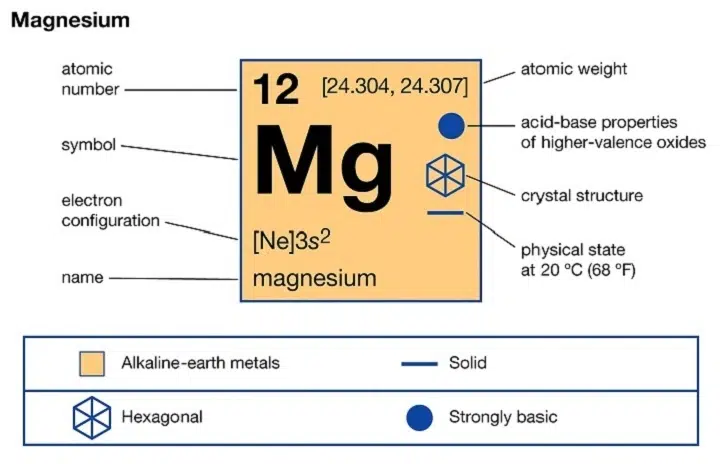
Magnesium is a chemical element with the atomic number 12. It belongs to Group 2 of the periodic table, also known as the alkaline earth metals. In its neutral form, magnesium is represented by the chemical symbol Mg. However, when magnesium forms an ion, it undergoes a significant transformation.
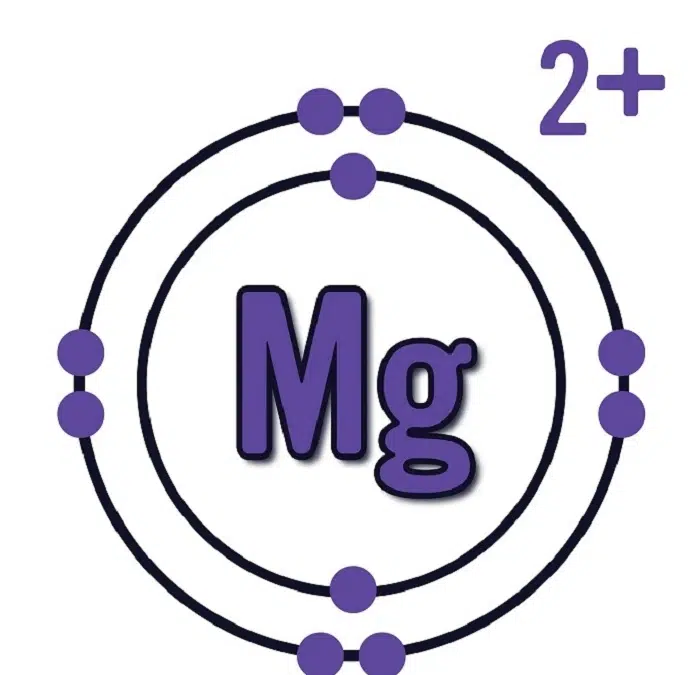
The magnesium ion is formed when a magnesium atom loses two electrons. This results in a divalent cation, carrying a 2+ positive charge. Therefore, the correct symbol of magnesium ion is:
Mg²⁺
This symbol is used universally across scientific disciplines and industries. The superscript 2+ indicates the ion’s charge, meaning it has lost two negatively charged electrons and carries a net positive charge.
Why Magnesium Forms a 2+ Ion
Magnesium has the electron configuration:
1s² 2s² 2p⁶ 3s²
The two electrons in the 3s orbital are its outermost (valence) electrons. Magnesium tends to lose these two electrons easily to achieve a stable noble gas configuration, similar to neon (1s² 2s² 2p⁶).
The ionization reaction can be represented as:
Mg → Mg²⁺ + 2e⁻
This process transforms neutral magnesium into a stable ion that participates in a wide variety of chemical reactions.
How to Write the Magnesium Ion Symbol Correctly
The correct format to represent the ion is:
- Elemental symbol: Mg
- Superscript charge: ²⁺ (not just + or ++)
Correct: Mg²⁺
Incorrect: Mg++, Mg+2, or mg2+
Using the correct ion symbol ensures clarity in chemical equations, lab reports, textbooks, and scientific communication.
Magnesium Ion in Chemical Reactions
Magnesium ions are key participants in ionic bonding and redox reactions. Here are a few examples:
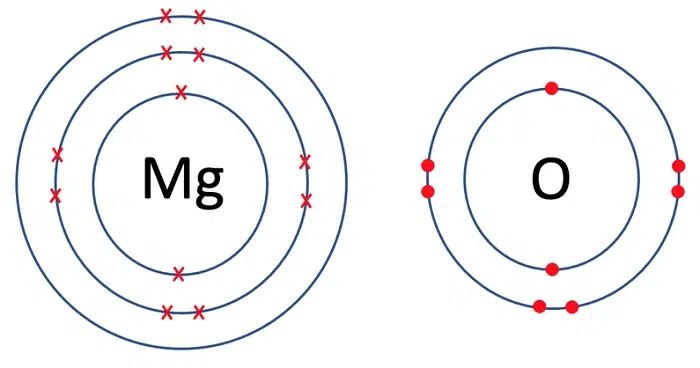
Ionic Compound Formation
Mg²⁺ + 2Cl⁻ → MgCl₂
Magnesium chloride is a common salt formed by combining magnesium ion with chloride anions.
Acid-Base Neutralization
Mg(OH)₂ → Mg²⁺ + 2OH⁻
Magnesium hydroxide dissociates into magnesium and hydroxide ions in solution.
Redox Reaction
Mg + 2H⁺ → Mg²⁺ + H₂↑
Magnesium displaces hydrogen in acids due to its higher reactivity.
These reactions showcase the critical role of magnesium ions in chemistry and industry.
Importance in Human Biology
The magnesium ion is vital for numerous biological processes in the human body. It is the fourth most abundant cation in the human body and is primarily stored in bones, muscles, and soft tissues.
Biological Functions of Mg²⁺:
- Acts as a cofactor for over 300 enzymes
- Regulates muscle and nerve function
- Stabilizes DNA and RNA structures
- Facilitates energy production via ATP binding
- Supports blood sugar and blood pressure control
Magnesium in Diet:
Good sources include leafy green vegetables, nuts, seeds, whole grains, and legumes. In the bloodstream, magnesium is mainly found as Mg²⁺, contributing to normal physiological functioning.
Magnesium Ion in Water and Soil
In environmental science, the presence of magnesium ion in water and soil is crucial for ecosystem health.
In Water:
- Contributes to water hardness
- Affects soap efficiency and scaling in pipes
- Present in groundwater and treated municipal supplies
In Soil:
- Essential for plant photosynthesis (part of the chlorophyll molecule)
- Deficiency can lead to yellowing leaves and poor crop yield
- Balanced Mg²⁺ levels support soil fertility
Monitoring Mg²⁺ levels in both systems helps maintain ecological balance and agricultural productivity.
Magnesium Ion in Medicine and Industry
Magnesium salts are widely used in medicine, typically containing the ion Mg²⁺. Common forms include:
- Magnesium sulfate (Epsom salt) – used in bath soaks and as a laxative
- Magnesium citrate – used for bowel prep
- Magnesium oxide – used in supplements and antacids
Industrial Uses:
- Metallurgy: Magnesium is used in alloys, especially in lightweight components
- Construction: As part of cement mixtures and fireproofing materials
- Electronics: Mg²⁺ ions are used in electrochemical cells and batteries
Digital Representation of the Symbol
To include the magnesium ion in digital content, the correct Unicode or HTML representation should be used:
- Unicode: Mg²⁺ (² = U+00B2, ⁺ = U+207A)
- HTML code: Mg²+
This ensures that your scientific writing appears correctly in web formats and academic publishing.
FAQs About the Symbol of Magnesium Ion
What is the correct symbol for magnesium ion?
The correct symbol is Mg²⁺, representing a magnesium atom that has lost two electrons.
Why does magnesium form a 2+ ion?
Magnesium has two electrons in its outer shell which it loses to achieve a stable electron configuration.
Where is Mg²⁺ found in the body?
It is found in bones, muscles, and blood and is essential for enzyme activity and energy metabolism.
Can the magnesium ion form compounds?
Yes, it forms ionic compounds with anions like Cl⁻, SO₄²⁻, and OH⁻, such as magnesium chloride (MgCl₂) and magnesium sulfate (MgSO₄).
How is Mg²⁺ important in water quality?
It contributes to water hardness and affects both human usage and industrial processes.
The symbol of magnesium ion, written as Mg²⁺, is a concise yet powerful representation of a critical element in both science and life. Whether in a chemistry lab, inside the human body, or flowing through our water systems, magnesium in its ionic form plays essential roles. Understanding this symbol is the first step in appreciating the diverse applications and biological importance of one of Earth’s most abundant and useful elements.